Seabird Sexing PCR Protocol using DNeasy Qiagen DNA extraction kits
Adapted for CCG use from Bento labs’ CHD1F/CHD1R Bird Sexing PCR Protocol
Note: Romney Edwards-Francis recommends concentrated (>100 ng/µL) DNA extracted from feathers using the HotSHOT protocol. The CCG has successfully run this protocol using the QIAGEN Blood & Tissue kit on a few drops of blood on a KimWipe, with pre-PCR concentrations of 0.3–1.2 ng/µL.
Gather the following materials:
- DNA sample (as little as ~0.5 ng/µL)
- 5x HOT Firepol master mix
- CHD1F/CHD1R primer mix
- Eppendorf tube, 1.5 mL
- PCR tubes, labeled
- Water, molecular grade
PCR Reaction Mix (20 µL per sample)
- 4 µL 5x HOT Firepol master mix
- 2 µL CHD1F/CHD1R primer mix
- 1–6 µL DNA template (try for at least ~1.5 ng)
- 8–13 µL PCR grade water (total volume 20 µL)
Protocol
Prepare PCR Master Mix in a 1.5 mL tube as follows:
- Multiply reagents by number of samples n, + 1 negative control, + ~10 % safety margin
- Add proportional amount 5x HOT Firepol master mix
- Add proportional amount CHD1F/CHD1R primer mix
- Add 6 µL PCR Master Mix to PCR tubes.
- Add 1-6 µL DNA template, or molecular-grade water for negative control, to PCR tubes.
- Add sufficient molecular-grade water to bring reaction volume to 20 µL.
- Vortex and spin down. Transfer to thermal cycler for PCR.
PCR program for CHD1F/CHD1R primers
Ramp Up:
- 94°C for 15mins (If using 5x HOT Firepol. Only 4mins if using 5x Firepol master mix)
8 cycles:
- 94 °C, 30 sec
- 57 °C-50°C, 45 sec (touchdown: reduce 1 °C/cycle for 8 cycles)
- 72 °C, 45 sec
27 cycles:
- 94 °C, 30 sec
- 50 °C, 45 sec
- 72 °C, 45 sec
- 72 °C, 5 min
- 12°C, ∞
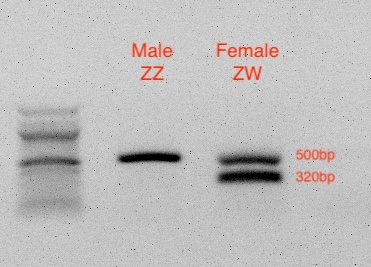
Analyze Your Results on a Gel
Compare your gel to this example result: You should see either 500bp for ZZ males or two bands at 500bp and 320bp for ZW females.